
Managing your soil’s pH is vital for healthy plants and a gorgeous lawn. When it’s outside of the recommended range, it can negatively affect nutrient availability, impacting plant growth.
I’ll explain how to change your soil pH, restoring healthy growth in your lawn. Acidic soil can be adjusted by adding lime or wood ash, raising the pH. If your soil is too alkaline, you can add sulfur, aluminum sulfate, or iron sulfate to bring the soil pH down.
As a soil scientist by education, I know a thing or two about soil pH. In grad school, I tested countless samples — probably in the thousands — to check soil pH and taught undergrads how to do it in soil fertility labs. Living in an arid Idaho climate, my soil tends to be alkaline (8.2 to 8.4), so I have to amend it to keep my lawn and garden thriving.
Understanding Soil pH
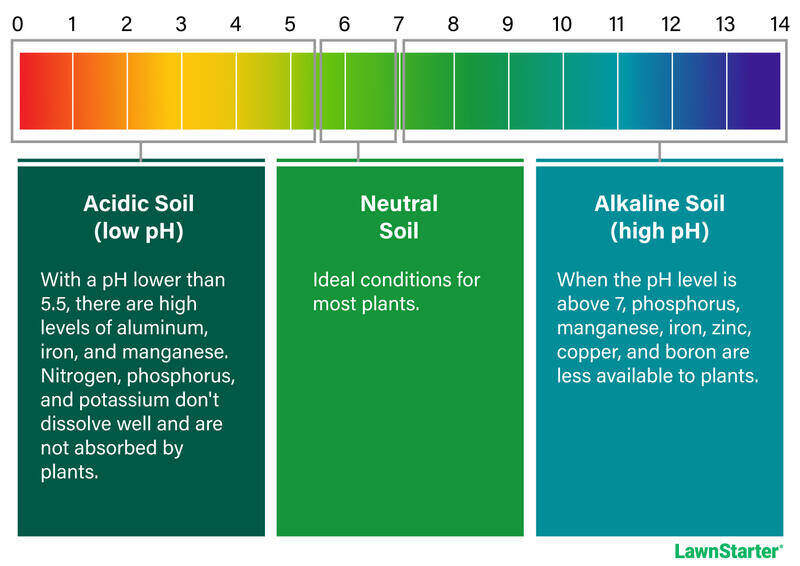
The pH level of your soil indicates its acidity or alkalinity. From a chemical perspective, it reveals your soil’s hydrogen ion (H+) concentration.
The soil pH scale ranges from 0 to 14, with neutral soil sitting at 7.0. When your soil has a higher concentration of H+ ions, the pH values fall below 7.0, creating acidic conditions. When fewer H+ ions are present — or there’s a higher concentration of hydroxyl ions (OH–) — the pH rises above 7.0, resulting in alkaline conditions.
Pro Tip: Mathematically speaking, the pH scale is logarithmic, not linear. When it changes by one unit — from 6.0 to 7.0 — there is a ten-fold change in the concentration of hydrogen ions. Soil with a pH of 6.0 is 10 times more acidic than soil with a pH of 7 and 100 times more acidic than soil with a pH of 8.
If chemistry isn’t your strong suit, hire a landscaping professional to help you achieve the optimal pH and get your lawn back on track.
The Role of Soil pH in Plant Growth
Soil pH is important because it highly impacts nutrient availability, which is critical for healthy lawns and plant growth. It can also increase the incidence of certain lawn diseases.
Soil pH Affects Nutrient Availability
When soil pH starts moving away from neutral, nutrients in the root zone can become unavailable for plant uptake or highly available in some cases.
- When plant essential nutrients are unavailable, your lawn and plants experience nutrient deficiencies. You’ll see stunted plant growth and maybe yellowing of plant tissues.
- When some nutrients become overly available, this can cause toxicity problems. Sometimes, too much of a good thing is bad, and the toxicities can damage or even kill your plants.
The tricky part about nutrient availability is that it doesn’t follow a consistent pattern across nutrients.
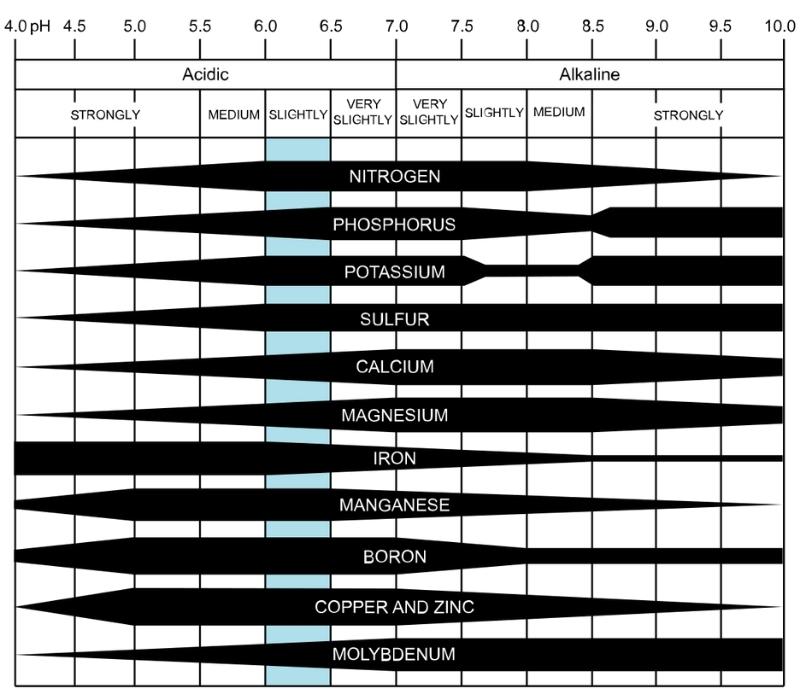
- As your pH drops, the soil becomes more acidic, and essential nutrients, including nitrogen, potassium, phosphorus, calcium, and magnesium, become less available. Iron, manganese, copper, and zinc become highly available.
- As pH increases and your soil becomes more alkaline, phosphorus, potassium, and sulfur become highly available. Nitrogen, calcium, magnesium, iron, and zinc become less available.
See Related: How Soil pH Affects Lawn Health
Soil pH Increases Disease Problems
When the pH starts moving too far from neutral, you may also see more problems with lawn diseases. As the pH drops:
- Acidic soils have more problems with Fusarium patch and red thread.
- Alkaline soils are at a higher risk for necrotic ring spot, take-all root rot, summer patch, fairy ring, Pythium blight, and powdery mildew.
Optimal Soil pH
When it comes to ideal soil pH values, it’s hard to narrow down exact numbers. It’s more important to find that sweet spot within a range.
- Oregon State recommendations 5.5 to 6.0 for lawns.
- Penn State University Extension says 6.2 to 6.8 is suitable for most plants, including garden veggies and ornamentals.
- The USDA’s Natural Resources Conservation Service says 6 to 7.5 is optimal for crop growth.
As a general recommendation, keep your soil pH between 6.0 and 7.0 (slightly acidic to neutral) for your lawn, garden, and the majority of ornamental plants.
Some acid-loving plants like rhododendrons, azaleas, and blueberries need more acidic soil — down between 4.5 and 5.5, which is lower than the recommended range — so many homeowners need to acidify their soil to grow them. I don’t even try to grow acid-loving plants because of my naturally alkaline soil. That level of acidity would be hard for me to reach and maintain.
Other plants don’t require pH values outside those ideal ranges but will tolerate acidic or alkaline conditions. Bahiagrass, bentgrasses, centipedegrass, perennial ryegrass, and tall fescue will grow in acidic conditions. Lavender and lilacs tolerate slightly moderate alkaline soils, so they’re staples in my yard.
How Do You Measure Your Soil’s pH?

When it comes time to check your soil’s pH, you have 3 options, ranging in price and convenience. You can take the DIY route or opt for professional analysis.
Three Ways to Test Your Soil
- The cheapest way is to buy a soil pH test kit and check it yourself. They’re typically under $10 and can be bought online and at many home improvement stores with a garden center. You’ll generally have results within a couple of minutes max, but in most cases, the results give you a range, say between 6.0 and 6.5, not 6.1, 6.2, 6.3, etc.
- The most expensive DIY method is to use a digital pH meter. These testers range from $10 to over $200 and can be bought online or at garden centers. Their results are narrower than test kits, but accuracy depends on the quality of the meter you buy and how well it’s calibrated.
- The most accurate method is to send a soil sample to a testing lab for analysis. It may take a few weeks to get results, but you’ll know your exact soil pH. Testing only soil pH usually costs $10-20. For $50 to $100, you can add different nutrient analyses and a soil test report that contains fertilizer recommendations.
Testing via your local Cooperative Extension is the cheapest way to get professional results and is even free in the off-season in some states.
My Tip: Save yourself the money, and don’t buy a cheap pH meter. To get an accurate pH reading, you need to spend at least $50 on a meter and buy the appropriate calibration buffers to ensure it works correctly. Buying an at-home test kit for $10 is just as good.
See Related:
How to Make Soil Less Acidic

Chemically speaking, an acidic soil has too many hydrogen ions floating around. To make it less acidic, add soil amendments containing base cations (typically calcium or magnesium) to replace the H+ ions.
Lime
You can use different products to raise a soil’s pH level, but lime, a calcium-based compound, is the most common method. As lime breaks down in the soil, components chemically react with the hydrogen ions, neutralizing soil acidity.
What are the best liming materials?
When choosing a lime to raise your soil pH, there are two primary choices: calcitic and dolomitic lime. They both come from finely ground limestone but are slightly different based on the parent material.
- Calcitic lime (commonly called agricultural lime) primarily contains calcium compounds: calcium carbonate, calcium hydroxide, and calcium oxide. This pure calcium formulation makes it ideal for soils with balanced magnesium levels that simply need pH adjustment.
- Dolomitic limes offer dual benefits because they contain significant amounts of magnesium carbonate and calcium compounds. This makes it the perfect choice for acidic soils suffering from magnesium deficiencies, addressing two problems with one application.
Note: Don’t use anything labeled as “hydrated” limestone. These products work quickly to correct soil pH, but their caustic properties pose safety concerns, making them very dangerous.
What to look for when buying lime?
Two factors contribute to a lime’s effectiveness: calcium carbonate equivalent (CCE) and particle fineness.
- CCE measures neutralizing ability compared to pure calcium carbonate. A higher CCE means greater effectiveness and reduced application rates, saving you money and time.
- Particle size significantly impacts how quickly a lime works. Limestone doesn’t readily dissolve in water, so a finer grind creates more surface area for soil interaction, speeding up the process.
Pro Tip: Add a mix of lime particle sizes to your soil. You’ll see faster results from the small, fine particles, and the larger particles help to stabilize soil acidity over time.
How long does lime take to work?
When applying lime to change soil pH, expect it to take up to 6 months before seeing significant changes. Unfortunately, chemical reactions take time, no matter which lime product you use. The full effect typically takes 2 to 3 years.
Mixing the lime into the soil well instead of letting it sit on the soil surface helps to speed up the reaction time.
How much lime to apply?
Figuring out how much lime to apply is tricky and involves some not-so-fun math. To get a number, you need to factor in soil pH, texture, buffering capacity, and the lime’s CCE.
As a very general recommendation, acidic soils that only need their pH bumped up slightly need between 20 and 50 pounds of calcium carbonate per 1,000 square feet; heavy clays with strong buffering capacity may need up to 100 pounds per 1,000 square feet.
My Tip: If your soil is too acidic, I highly recommend not just guessing and throwing lime down. Go outside, take a soil sample, and send it off for analysis. The lab will test your soil, determine the pH and buffering capacity, and tell you exactly how much lime to add per 1,000 square feet to hit your target pH.
Need in-depth information on liming your lawn? Read more:
- How to Tell If Your Lawn Needs Lime (6 Signs)
- Lime for Lawns: Why, When, and How to Lime a Lawn
- Can You Apply Lime and Fertilizer to Your Lawn at the Same Time?
Wood Ash
The ash left from burning wood is high in the base cations calcium and potassium and has been used to amend soil pH back to the 1700s. The carbonates that form react with H+ ions, neutralizing the acidity and raising the soil pH.
It can take 2 to 4 times as much wood ash (depending on its calcium carbonate equivalent) to raise the soil pH, but it works faster since it’s more water-soluble.
Oyster Shell Meal
Finely ground oyster shells offer a natural and sustainable alternative to traditional agricultural lime. This organic soil amendment primarily contains calcium carbonate, providing a long-lasting, slow-release calcium source that benefits your soil for several years.
Oyster shell meal works more gradually than standard lime. Noticeable improvements may appear within weeks of application, but it will take several months to see significant benefits.
How to Make Soil More Acidic

Photo Credit: Leiem / Wikimedia Commons / CC BY-SA 4.0
Amending an alkaline soil to make it more acidic is more difficult than raising the pH to make it more alkaline, especially if working with clay soil, but it is doable. Instead of adding amendments to reduce hydrogen ions, you’ll add sulfur products to increase the concentration.
My Tip: Ammonium fertilizers can make soils more acidic, but they aren’t a good choice for making drastic soil pH changes. You’d need large amounts to make a significant difference, so they are better suited to help maintain more acidic conditions once a desired pH is reached.
What kind of sulfur is good for lowering soil pH?
Elemental sulfur, aluminate sulfate, and iron sulfate are the most common amendments homeowners and gardeners use to lower soil pH.
- Elemental sulfur is the safest and most economical choice but the slowest. Since it requires moisture and soil bacteria to break the sulfur into sulfuric acid, it typically takes 3 to 6 months to see results. Like lime fineness, smaller particles work quicker than larger ones.
- Aluminum sulfate changes soil pH quickly because it doesn’t need soil bacteria, only water to form sulfuric acid. Its ability to adjust soil pH quickly makes it a good option for ornamental plants, like hydrangeas, that need acidic soil to turn flowers blue. Since aluminum can be toxic at high levels, use it cautiously for your lawn or veggies.
- Iron sulfate works slower than aluminum sulfate and faster than elemental, making it a good middle-ground option. It adds essential iron, helping plant growth, but you need about 8 times more than elemental sulfur to significantly reduce pH.
Note: Sulfuric acid is sometimes used in agriculture to lower soil pH, but homeowners should avoid using it. It is incredibly caustic and corrosive, making it dangerous, even for trained professionals.
Personal Experience: My soil typically has an unamended pH of 8.2 to 8.4. I haven’t seen enough issues with the higher pH to warrant the work needed to adjust it for my lawn. But I do use a mix of elemental sulfur and iron sulfate on my garden soil.
I use elemental sulfur where I can till the soil in the spring, working it into the ground before planting. I scatter iron sulfate in my raspberry patch where I don’t work the ground; I water it in well, so it goes to work quicker than the elemental, and it gives an extra boost of iron to my deficient soil.
How much sulfur should I apply to change the soil pH?
To drop your soil’s pH by about half a point, use 0.5 to 1.0 pounds of elemental sulfur for every 1,000 square feet of your garden or lawn. Keep in mind that soil texture matters significantly — sandy soil needs less sulfur to get the job done, while heavy clay soils need more material to achieve the same pH change.
That said, paying for a professional soil test is really the best move — the lab will analyze your specific soil and tell you exactly how much sulfur to apply for perfect results, taking all the guesswork out of it.
Tips for Changing Your Soil pH
- Limit lime application to a maximum of 50 pounds per 1,000 square feet at a time.
- When possible, add soil amendments when the ground is bare and plants aren’t growing.
- For best results, lime soils in the fall to give them ample time to work before spring planting.
- Use sulfur-based products in the spring after soil temps start rising.
- Spread half of the product walking in one direction (North to South or East to West), and then apply the other half at a right angle for the best coverage.
- When feasible, work soil amendments into the ground.
- Always water thoroughly after applying products.
- If sulfur comes in contact with your plants’ leaves, rinse it off immediately to prevent damage to the foliage.
- Test soil pH every 3 months after amending to track progress and make necessary adjustments.
What Affects Soil pH?

Photo Credit: Shutterstock
Some factors, like the type of parent material that lies beneath your topsoil and the kind of soil you have, give your soil its “starting” pH. However, this pH can change over time because of other influential factors, including rainfall and soil amendments like fertilizer. This is why even after you’ve changed it to get it where you want it, the pH can move out of that recommended range.
Parent Materials Impact Soil pH
Soil is formed by big rock chunks found deep in the ground. As these parent materials break down, their mineral content determines the resulting soil’s pH.
- Limestone parent materials contain high amounts of calcium carbonate, resulting in alkaline soil.
- Granite and shale parent materials release hydrogen ions as they weather, creating acidic soils.
Soil Type Helps Determine pH
Your soil type also plays a role in pH, mainly because the soil particle size affects how soil attracts and binds with positively charged molecules.
Sandy soils tend to be naturally acidic. This occurs because sand is the largest particle size, so it doesn’t hold onto base cations as tightly as smaller soil particles. Larger base cations like calcium and magnesium are easily washed away from rain or irrigation, leaving smaller hydrogen ions contributing to a lower pH level.
On the other hand, clays tend to be alkaline. The small soil particles have a huge surface area, allowing them to strongly attract and tightly hold base cations like calcium (Ca2+) and magnesium (Mg2+), which have a larger positive charge than hydrogen. With fewer hydrogens in the soil, the pH is higher.
The affinity for calcium and magnesium to tightly bind to clay particles makes adjusting a clay soil’s pH level harder than a sandy soil. Calcium and magnesium are held so tightly to the clay particles that they are hard to “knock off” and replace with H+ to lower the pH.
Rainfall Plays a Part in Soil Acidity
Areas with rainy climates typically have acidic soils. The lower pH occurs because rain leaches base cations like calcium and magnesium out of the soil, leaving hydrogen behind to drop the pH value.
With this in mind, it’s easy to understand why soils along the coasts and in the eastern half of the U.S. are more acidic. Arid climates like Arizona and here in Idaho have less rain and less leaching, so soils are typically more alkaline.
Soils also tend to be more acidic in urban environments and heavy manufacturing areas because of acid rain.
Note: Overwatering your lawn impacts the soil pH the same way as heavy watering. To make sure you’re watering correctly, you can read more in our articles:
Fertilizer and Organic Materials Can Change Soil pH
Fertilizer and other soil amendments are great for helping keep your lawn and garden healthy, but adding them can affect the soil’s pH. The University of California Division of Agriculture and Natural Resources gives the following examples:
- Fertilizers that use ammonium-based nitrogen sources can acidify the soil. Bacteria in the soil break the ammonium down into nitrate, releasing H+ and lowering the pH. Fertilizers with nitrate-based nitrogen sources can make the soil more alkaline. When the roots absorb nitrate, it is usually accompanied by a hydrogen ion. Less H+ in the soil increases the pH.
- Animal manure can raise pH because it contains the base cations calcium and magnesium.
- Potassium fertilizers and compost don’t typically change soil pH unless the compost contains a lot of pine needles or citrus peels.
Note: Compost doesn’t necessarily change the soil pH, but adding compost can help buffer future pH changes.
FAQ About Modifying Soil pH
Use a finely ground calcitic lime with a high CCE for fast pH increases. Mix it thoroughly into the top few inches of your soil and keep the ground well-watered to activate the lime. To bring the pH down in a hurry, aluminum sulfate is your best bet for quick results.
I’m sorry to say, but coffee grounds won’t lower your soil pH in the long run. Their pH is 6.5 to 6.8, putting them only slightly below neutral. You might see a slight decrease in pH when you add them to alkaline soils, but the effect doesn’t last.
Not all peat moss can help acidify soil, but some can be used. As Joe Hannah from Iowa State University points out, Canadian sphagnum peat moss has a significantly acidic pH (around 3.0 to 4.5), making it effective for lowering soil pH.
Let the Lawn Care Pros Help
Changing your soil pH is an essential step towards having a thriving lawn and garden, but it’s just one piece of the bigger puzzle. Even after you achieve the optimal pH, regular maintenance — like mowing, fertilizing, and aerating — is key to keeping your landscaping healthy and vibrant.
If you need a hand with your lawn care or gardening, reach out! Lawn Starter connects you with local professionals who can handle everything from weed control to fertilization. You can sit back and enjoy a lush, green yard without the hassle.
Sources:
- “Fertilizers and Soil pH.” University of California Division of Agriculture and Natural Resources.
- “How To Change Your Soil’s pH.” By Joe Hannan, field specialist. Iowa State University.
- “Keeping pH in the Right Range is Essential.” By Kym Pokorny, master gardener. Oregon State University.
- “Soil Health – pH.” Natural Resources Conservation Service.
- “Understanding Soil pH.” By Mary Jo Gibson, master gardener. Penn State Extension.
Main Image Credit: Paul Maguire / Adobe Stock