
A neutral soil pH safeguards beneficial nutrients made available to your plants. High or low pH levels can diminish plant nutrients and introduce toxic soil elements. Let’s dig deeper into how soil pH affects lawn health.
pH is the measure of soil acidity or alkalinity. Maintaining a balanced soil pH is essential to your grass and native plants. Even with proper watering and fertilizing, your lawn’s health will deteriorate if the soil pH is lower than 6 or higher than 7. We’ll expand on the negative effects of soil pH extremes, factors that affect soil pH, and how to test and adjust the soil pH.
What Is Soil pH?
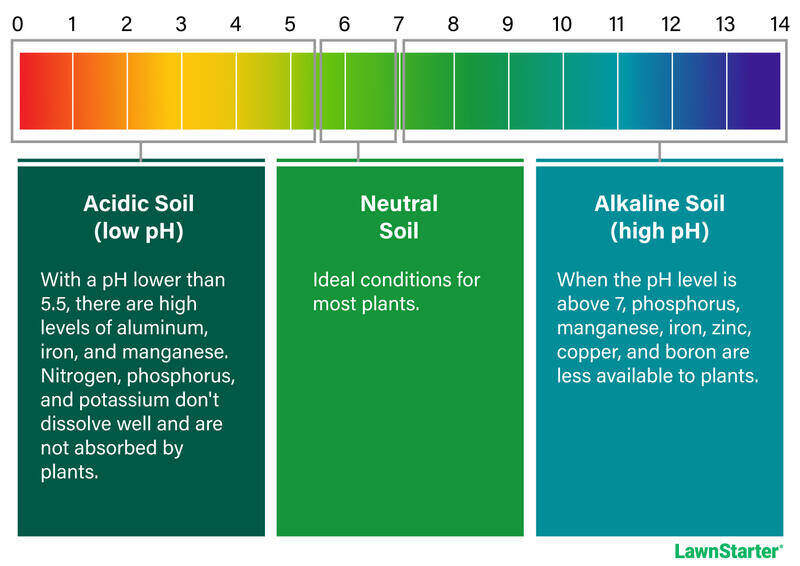
Soil pH reveals whether your soil is basic (alkaline), neutral, or acidic. The pH scale ranges from 0 to 14, with 7 being a neutral state that’s neither acidic nor basic. Most turfgrasses and plants thrive at a pH range between 6 to 7.
Effects of Soil pH On Your Lawn’s Health
The soil pH directly affects the growth and quality of your grass and native plants. It influences the chemical form of soil microbes and other beneficial elements needed for a healthy lawn. Extremes in soil pH levels have two negative effects on your lawn’s health:
- Decreased availability of plant nutrients
- Increased concentration of plant-toxic minerals
Decreased Availability of Plant Nutrients
Soil pH influences nutrient availability for turf and other plants. When the pH is too high or too low, the nutrients remain unavailable no matter how much fertilizer you add. Overall, a soil pH that’s no lower than 5.5 and not higher than 7 provides ideal conditions for a healthy lawn.
Pro Tip: Some grasses can tolerate a soil pH as low as 5.5 (such as centipedegrass). However, most grasses prefer a pH range between 6 and 7.
Increased Concentration of Plant-Toxic Minerals
Acidic soils with a pH lower than 5.5 contain increased levels of toxic elements such as aluminum (Al), zinc (Zn), and iron (Fe). Beneficial plant nutrients such as potassium (K), magnesium (Mg), and calcium (Ca) are significantly reduced.
Alkaline soils with a pH higher than 7, more plant nutrients such as boron (B) and manganese (Mn) become deficient.
Factors Affecting Soil pH
Over time, soil pH level tends to decrease (more acidic). This occurs when basic cations such as potassium and sodium are replaced by acid-forming cations. Here’s how the soil pH decreases:
- Rain or snowfall: Water passing through the soul leaches basic soil nutrients and replaces them with acidic elements such as iron and aluminum.
- Fertilizers: Application of fertilizers, especially those containing ammonium or nitrogen, can speed up soil acidification.
- Organic matter breakdown: Decomposition of organic matter such as pine needles, plant litter, and vegetation can lower the pH and acidify the soil. This is a common issue in heavily forested areas.
- Hydrogen ions: Some legume plants release hydrogen ions through their roots to maintain the electrochemical balance within their tissues. Hydrogen ions increase the soil’s acidity level.
Soil pH can also increase over time. Alkaline soils will hinder your plants’ growth rate just like acidic soils. So how does the soil pH increase?
- Climate: States such as Montana that have an arid, dry climate tend to have soils with a higher pH (alkaline) than areas with regular rainfall.
- Excess compost: Soils that receive excess compost such as manure have higher pH levels due to the build-up of base cations.
- Over-liming: Applying too much lime to an acidic soil with minimal watering causes a counter-effect that makes the soil more basic.
How to Test Your Soil pH

Testing the soil pH determines whether your soil is acidic or basic (alkaline). There are two main approaches to soil pH testing: Professional testing at a lab and DIY testing.
Professional Testing
This isn’t as simple as digging up a soil sample and shipping it by post. You need to collect 10 to 15 samples from fertilizer-free areas to mix into a macro-mixture that represents your lawn.
To dig straight down into the soil, grab a shovel and dig a hole that’s about 6 to 12 inches deep. Remove any organic matter such as worms and thatch.
Use a trowel to mix the samples in a bucket, crushing clumps until you have smooth soil. Take 1 or 2 cups of soil and let it dry on the newspaper without getting wet.
Once it’s dry, place the sample in a container (or plastic bag) and follow the local lab’s mailing instructions. But how do you locate a soil testing laboratory?
Most cooperative extension offices offer affordable soil testing services. Check with your local Cooperative Extension office to learn how and where to send a soil sample.
DIY Testing
There are various DIY soil pH testing kits to choose from, especially through online retailers such as Amazon and Walmart. Whether you opt for test strips or sensory testers, follow the manufacturer’s instructions to get accurate results.
The best time to perform a DIY soil test is in spring or early fall, before you’ve treated or fertilized your lawn. DIY soil tests often give mixed results, so professional testing is best for accurate results.
Here are detailed steps on how to test the soil pH of your lawn, whether it’s DIY or professional testing.
FAQ About Soil pH
What are the pros and cons of professional soil pH testing?
Sending a sample to a professional soil testing lab may not always be the most convenient option. Let’s explore its pros and cons:
Pros of professional soil testing:
- Accurately determine your soil’s nutrient levels
- Accurately determine your soil’s pH levels
- Professionals will recommend proper use of soil amendments and fertilizers to fix soil issues
- Reasonably affordable
Cons of professional soil testing:
- Multiple testing can be costly
- May take weeks before you obtain results
- Not as convenient as direct in-person testing
What are the macro- and micro-nutrients affected by soil pH extremes?
Extreme levels in the soil pH can significantly lower the amount of plant nutrients in the soil. Here are the main ones:
Macronutrients:
- Sulfur
- Magnesium
- Calcium
- Potassium
- Nitrogen
- Phosphorus
Micronutrients:
- Zinc
- Copper
- Boron
- Chlorine
- Iron
- Manganese
What happens when the soil pH is lower than 5?
With a soil pH lower than 5, plants will be stunted and prone to root diseases. These include blossom end rot, grass root rot, dollar spot, and red thread.
Hire a Pro to Nurture Your Lawn
Keeping your soil in a neutral pH is like building your home’s foundation. If the soil chemical levels are imbalanced, then any following lawn treatments are rendered fruitless.
Lawn mowing is another task to perform before treatments like aeration and overseeding. For more lawn activities and less lawn chores, hire a local lawn care professional to mow your turf and preserve its green aesthetic.
Main Photo Credit: Backyard Boss / Flickr / CC BY 2.0 DEED